

June 2021 Potentially, we believe, to be First in a New Class of Antibiotics Since the 1980s 1,2 World Health Organization & U.S. Centers for Disease Control and Prevention Priority Pathogens 1,3 Readiness for the next Infectious Disease Pandemic: Antibiotic Resistance Disclosures 1 Centers for Disease Control, November 2019; 2 Wei - Chu Xu, et al., Bioorganic & Medicinal Chemistry, June 2018; 3 World Health Organization, December 2019 There is no guarantee that any specific objective will be achieved. Acurx Pharmaceuticals, LLC products are in development, and the re is no guarantee that this development will have a successful outcome. Clinical trials are in the early stages. Pre - Clinical trials are tested on animals. Investments may be illiquid, highly speculative and there is risk of the total loss of your investment. See disclosures at the beginning. Issuer Free Writing Prospectus Filed Pursuant to Rule 433 Registration No. 333 - 256516

Disclosures DISCLAIMER The Company has filed a registration statement, including a preliminary prospectus, with the SEC (File No . 333 - 256156 ) in connection with the offering to which this presentation relates . Sales of the securities of the Company offered pursuant to the registration statement may not be made or offers for such securities accepted prior to the registration statement becoming effective . Before you invest, you should read the registration statement, the preliminary prospectus included within the registration statement and other documents the Company has filed with the SEC for more complete information about the Company and this offering . You can obtain a copy of the preliminary prospectus for free by visiting EDGAR on the SEC website at www . sec . gov . Alternatively, the Company will arrange to send you the preliminary prospectus, which you may request by emailing davidluci@acurxpharma . com . This presentation may not be reproduced, forwarded to any person or published, in whole or in part . The Company is not soliciting offers to buy securities of the Company in any jurisdiction where the offer or sale is not permitted . FORWARD LOOKING STATEMENTS This presentation contains forward - looking statements as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, development plans, regulatory activities, anticipated milestones, product candidate benefits, competitive position, business strategies, objectives of management, potential growth opportunities, potential market size, possible or assumed future results of operations, projected costs and use of proceeds . In some cases, forward - looking statements can be identified by terms such as “may,” “will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “could,” “intent,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions . All statements other than statements of historical facts contained in this presentation are forward - looking statements . Acurx Pharmaceuticals, LLC (the ‘ Company”) may not actually achieve the plans, intentions or expectations disclosed in these forward - looking statements . Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward - looking statements as a result of various factors, including : uncertainties inherent in the initiation and completion of preclinical studies and clinical trials and clinical development of the Company’s product candidates, including adverse results in our clinical development processes ; whether results from one clinical trial will be predictive of the results of future trials and whether preliminary data from our clinical trials will be predictive of final results from such trials ; decisions made by the U . S . Food and Drug Administration and other regulatory authorities with respect to the development and commercialization of our products ; availability of funding sufficient for the Company’s foreseeable and unforeseeable operating expenses and capital expenditure requirements ; our ability to obtain, maintain and enforce intellectual property and other proprietary rights for our product candidates ; our ability to implement our strategic plans ; and other factors discussed in the “Risk Factors” section of the Company’s filings with the Securities and Exchange Commission (“SEC”) in the future . The forward - looking statements included in this presentation represent the Company’s views as of the date of this presentation . The Company anticipates that subsequent events and developments will cause its views to change . However, while the Company may elect to update these forward - looking statements at some point in the future, it specifically disclaims any obligation to do so . These forward - looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this presentation . Disclosures Acur x R&D Pipeline Disclosures There is no guarantee that any specific objective will be achieved. Acurx Pharmaceuticals, LLC products are in development, a nd there is no guarantee that this development will have a successful outcome. Clinical trials are in the early stages. Pre - Clinical trials are tested on animals. Investments may be illiquid, highly speculative and there is risk of the total loss of your investment. See disclosures at the beginning. Disclosure Acur x 2
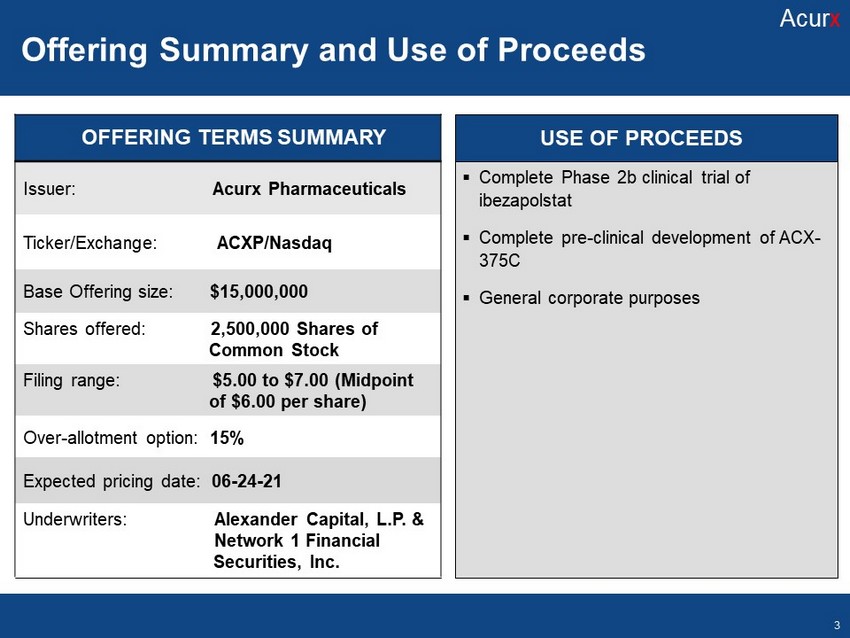
Issuer: Acurx Pharmaceuticals Ticker/Exchange: ACXP/Nasdaq Base Offering size: $15,000,000 Shares offered: 2,500,000 Shares of Common Stock Filing range: $5.00 to $7.00 (Midpoint of $6.00 per share) Over - allotment option: 15% Expected pricing date: 06 - 24 - 21 Underwriters: Alexander Capital, L.P. & Network 1 Financial . Services , Inc. USE OF PROCEEDS OFFERING TERMS SUMMARY USE OF PROCEEDS Offering Summary and Use of Proceeds Acur x 3 USE OF PROCEEDS ▪ Complete Phase 2b clinical trial of ibezapolstat ▪ Complete pre - clinical development of ACX - 375C ▪ G eneral corporate purposes

Corporate: Co - Founded July 2017 to develop a new class of antibiotics to address global crisis of antimicrobial resistance in Gram - positive bacteria; initial target - CDI Novel Mechanism of Action: DNA polymerase IIIC inhibitor ▪ Previously unexploited scientific target ▪ Positioned, we believe, to be first - line treatment for CDI Unmet medical need : CDC classifies CDI as an urgent threat requiring new antibiotic development. Existing antibiotics used to treat CDI marketed for decades have recurrent infection of 20% to 40% 1 and antibiotic resistance (metronidazole) necessitating development of new antibiotics to treat CDI Phase 2a Success/Phase 2b Ready: Ibezapolstat recently completed Ph2a clinical trial in patients with CDI – 100% cure rate and 100% sustained clinical cure 30 days after EOT COVID - 19 brings infectious disease threats into worldwide spotlight as policy makers look to bring antibiotics’ development back to the U.S. Disclosures 1 Johnson,et al : Sustained Clinical Response as an Endpoint in Treatment Trials of Clostridium difficile - Associated Diarrhea, Antimicrobial Agents and Chemotherapy, August 2012 Executive Summary Acur x 4

Disclosures ▪ Antimicrobial resistance occurs when microorganisms (bacteria, fungi, viruses, and parasites) change when exposed to antimicrobial drugs. ▪ WHO Director - General recently warned that growing antimicrobial resistance is as dangerous as the ongoing COVID - 19 pandemic threatens to unwind a century of medical progress and leave us defenceless against infections that today can be treated easily.* ▪ Acurx believes that the high level of awareness of COVID - 19 viral pandemic sets the stage for higher awareness, interest and importance of our readiness for an antimicrobial resistance pandemic. 1 Prestinaci, et al, Antimicrobial Resistance : a Global Multifaceted Phenomenon, Pathog Gob Health, October 2015 ; 2 CDC Antibiotic Resistance Threats in the U . S . , 2019 , Atlanta, U . S . Department of Health and Human Services, CDC, 2019 ; 3 WHO, 2019 Antibacterial Agents in Clinical Development ; * CTV News, 23 Nov 2020 Antimicrobial Resistance Acur x ▪ Antibiotic resistance not only a U.S. problem — it is a global crisis 2 ▪ New antibiotics are an important piece of the fight against antibiotic resistance ▪ Clinical pipeline remains insufficient to tackle the challenge of increasing emergence and spread of antimicrobial resistance 3 5
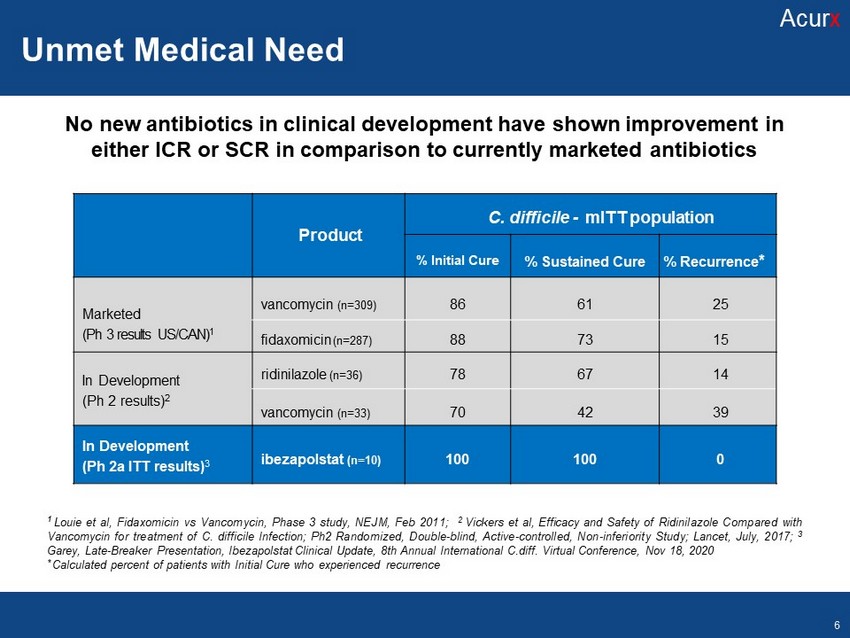
Mechanisms of Action Disclosures Disclosures No new antibiotics in clinical development have shown improvement in either ICR or SCR in comparison to currently marketed antibiotics P roduct C. d iff icile - mITT population % Initial Cure % Sustained Cure % Recurrence * Marketed (Ph 3 results US/CAN) 1 v ancomycin (n=309) 86 61 25 f idaxomicin (n=287) 88 73 15 In D e v elopme n t (Ph 2 results) 2 r idinilazole (n=36) 78 67 14 v ancomycin (n=33) 70 42 39 In Development (Ph 2a ITT results) 3 ibezapolstat (n=10) 100 100 0 1 Louie et al, Fidaxomicin vs Vancomycin, Phase 3 study, NEJM, Feb 2011 ; 2 Vickers et al, Efficacy and Safety of Ridinilazole Compared with Vancomycin for treatment of C . difficile Infection ; Ph 2 Randomized, Double - blind, Active - controlled, Non - inferiority Study ; Lancet, July, 2017 ; 3 Garey, Late - Breaker Presentation, Ibezapolstat Clinical Update, 8 th Annual International C . diff . Virtual Conference, Nov 18 , 2020 * Calculated percent of patients with Initial Cure who experienced recurrence Unmet Medical Need Acur x 6
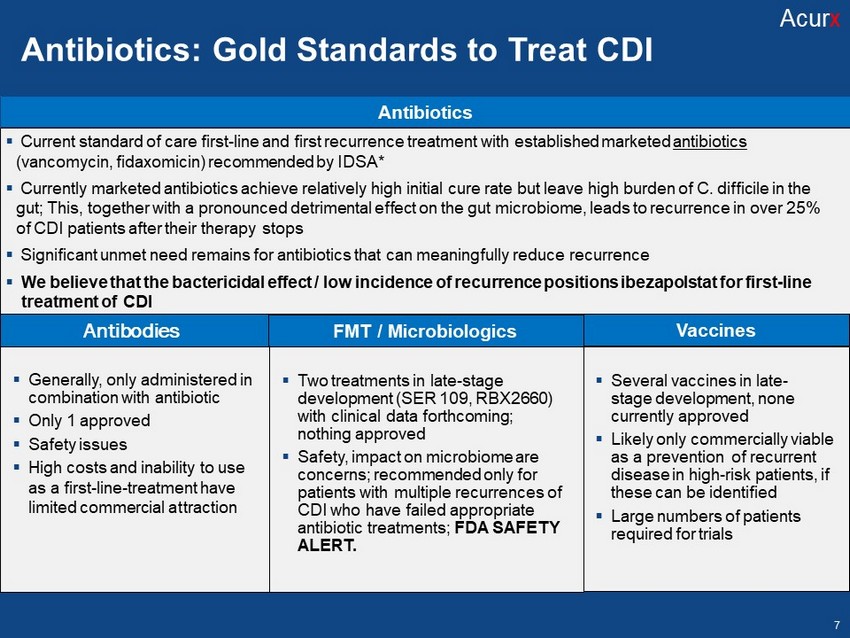
Disclosures FMT / Microbiologics Vaccines Antibiotics ▪ Current standard of care first - line and first recurrence treatment with established marketed antibiotics (vancomycin, fidaxomicin) recommended by IDSA * ▪ Currently marketed antibiotics achieve relatively high initial cure rate but leave high burden of C. difficile in the gut ; This, together with a pronounced detrimental effect on the gut microbiome, leads to recurrence in over 25% of CDI patients after their therapy stops ▪ Significant unmet need remains for antibiotics that can meaningfully reduce recurrence ▪ We believe that the bactericidal effect / low incidence of recurrence positions ibezapolstat for first - line treatment of CDI Antibiotics: Gold Standards to Treat CDI Acur x 7 ▪ Generally, only administered in combination with antibiotic ▪ Only 1 approved ▪ Safety issues ▪ High costs and inability to use as a first - line - treatment have limited commercial at traction Antibodies ▪ Two treatments in late - stage development (SER 109, RBX2660) with clinical data forthcoming; nothing approved ▪ Safety, impact on microbiome are concerns; recommended only for patients with multiple recurrences of CDI who have failed appropriate antibiotic treatments; FDA SAFETY ALERT. ▪ Several vaccines in late - stage development, none currently approved ▪ Likely only commercially viable as a prevention of recurrent disease in high - risk patients, if these can be identified ▪ Large numbers of patients required for trials
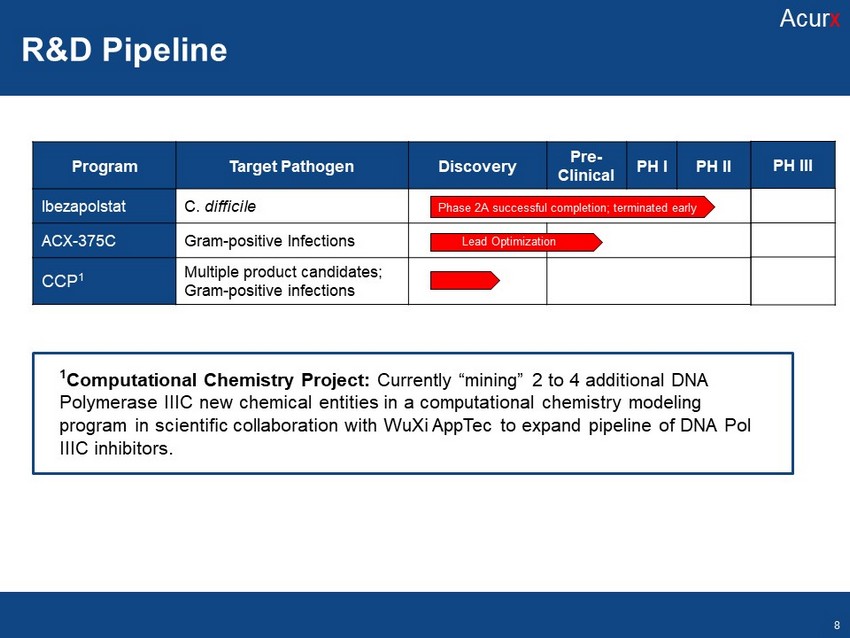
Acur x R&D Pipeline 1 Computational Chemistry Project: Currently “mining” 2 to 4 additional DNA Polymerase IIIC new chemical entities in a computational chemistry modeling program in scientific collaboration with WuXi AppTec to expand pipeline of DNA Pol IIIC inhibitors. Disclosures Disclosures R&D Pipeline Acur x Program Target Pathogen Discovery Pre - Clinical PH I PH II Ibezapolstat C. difficile ACX - 375C Gram - positive Infections CCP 1 Multiple product candidates; Gram - positive infections Lead Optimization Phase 2A successful completion; terminated early 8 PH III
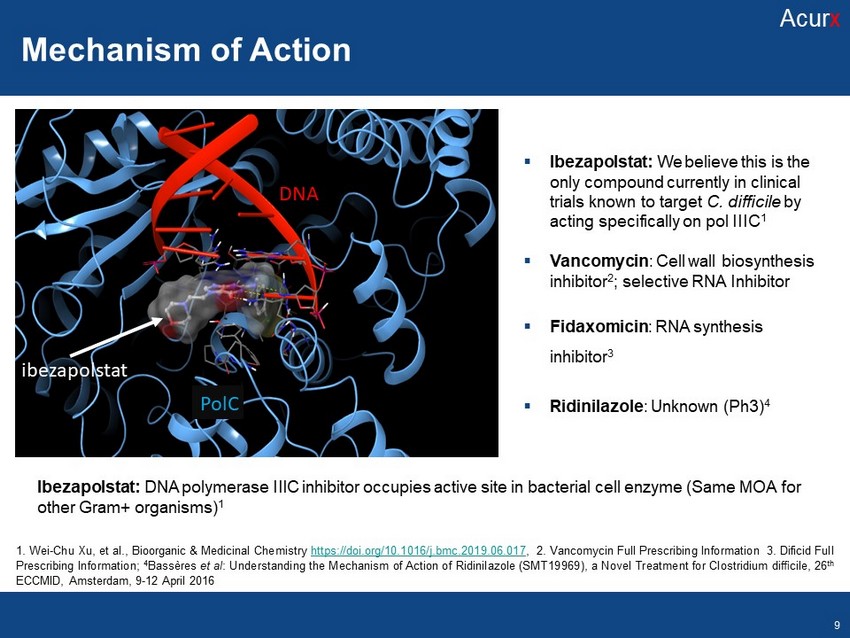
Mechanisms of Action ▪ Ibezapolstat: We believe this is the only compound currently in clinical trials known to target C. difficile by acting specifically on pol IIIC 1 ▪ Vancomycin : Cell wall biosynthesis inhibitor 2 ; selective RNA Inhibitor ▪ Fidaxomicin : RNA synthesis inhibitor 3 ▪ Ridinilazole : Unknown (Ph3) 4 Ibezapolstat : DNA polymerase IIIC inhibitor occupies active site in bacterial cell enzyme (Same MOA for other Gram+ organisms) 1 ibeza polstat PolC DNA Disclosures Disclosures 1 . Wei - Chu Xu, et al . , Bioorganic & Medicinal Chemistry https : //doi . org/ 10 . 1016 /j . bmc . 2019 . 06 . 017 , 2 . Vancomycin Full Prescribing Information 3 . Dificid Full Prescribing Information ; 4 Bassères et al : Understanding the Mechanism of Action of Ridinilazole (SMT 19969 ), a Novel Treatment for Clostridium difficile, 26 th ECCMID, Amsterdam, 9 - 12 April 2016 Mechanism of Action Acur x 9
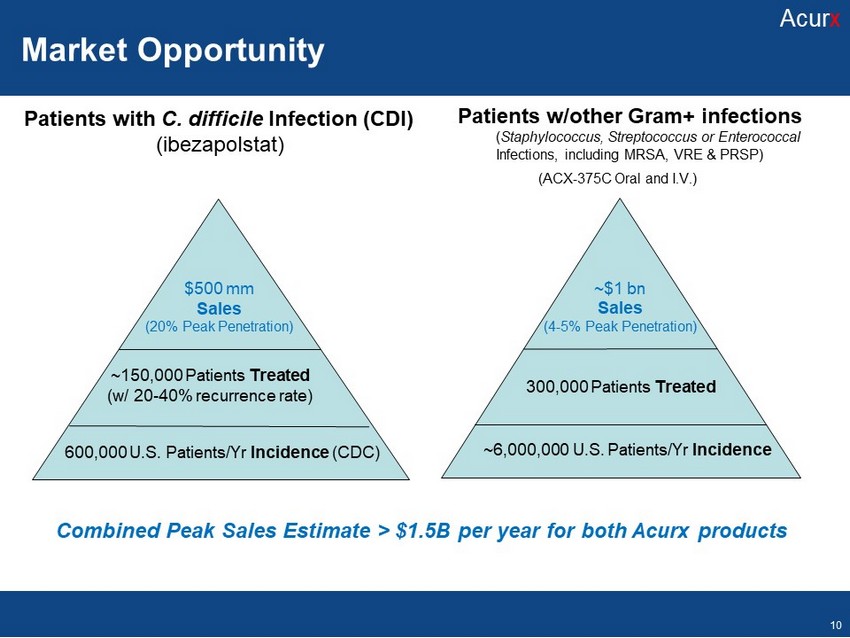
Patients with C. difficile Infection (CDI) ( ibezapolstat ) $500 mm Sales (20% Peak Penetration) ~150,000 Patients Treated (w/ 20 - 40% recurrence rate) 600,000 U.S. Patients/ Yr Incidence (CDC) Patients w/other Gram+ infections ( Staphylococcus, Streptococcus or Enterococcal Infections, including MRSA, VRE & PRSP) (ACX - 375C Oral and I.V.) ~$1 bn Sales (4 - 5% Peak Penetration) 300,000 Patients Treated ~6,000,000 U.S. Patients/ Yr Incidence Combined Peak Sales Estimate > $1.5B per year for both Acurx products Acur x Market Opportunity Acur x 10
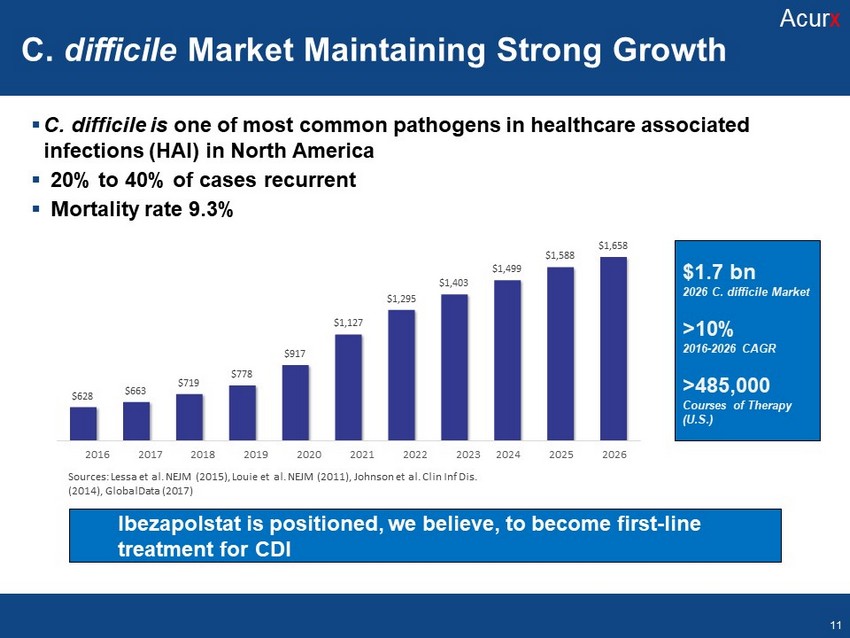
▪ C. difficile is one of most common pathogen s in healthcare associated infections (HAI) in North America ▪ 2 0 % to 40% of cases recurrent ▪ Mortality rate 9.3% $628 $663 $719 $778 $917 $ 1 ,1 2 7 $ 1 ,2 9 5 $ 1 ,4 0 3 $ 1 ,4 9 9 $ 1 ,5 8 8 $ 1 ,6 5 8 2016 2017 2018 2019 2020 2021 2022 2023 Sources: Lessa et al. NEJM (2015), Louie et al. NEJM (2011), Johnson et al. Clin Inf Dis. (2014), GlobalData (2017) 2024 2025 2026 Ibezapolstat is positioned , we believe, to become first - line treatment for CDI C. Difficile Market maintaining strong growth >1% 2016 - 2026 AGR > .) Acur x C. difficile Market Maintaining Strong Growth Acur x $1.7 bn 202 6 C. difficile Market >10% 2016 - 2026 CAGR >485,000 Courses of Therapy (U.S.) 11

ACX - 362E Highlights ▪ Patients with mild to moderate CDI treated with orally administered ibezapolstat given 450mg twice daily for 10 days ▪ A ll 10 patients (100%) met the study's primary and secondary efficacy endpoints of Initial Cure and Sustained Cure ▪ Ibezapolstat very well tolerated; no treatment - related SAEs ▪ Meeting the treatment goals of eliminating the infection with an acceptable adverse event profile allowed early termination of the Phase 2a trial and advancement to the Phase 2b trial ▪ Phase 2b clinical trial: 64 patients in 2 arms treated for 10 days; ▪ 32 patients will receive ibezapolstat; 32 patients will receive vancomycin, which is the current standard of care ▪ US Exclusivity: Patented to September 2030; Potential Regulatory Exclusivity: 10 years from FDA marketing approval (QIDP and Fast Track) Disclosures Disclosures Ibezapolstat : Phase 2a Success Acur x 12
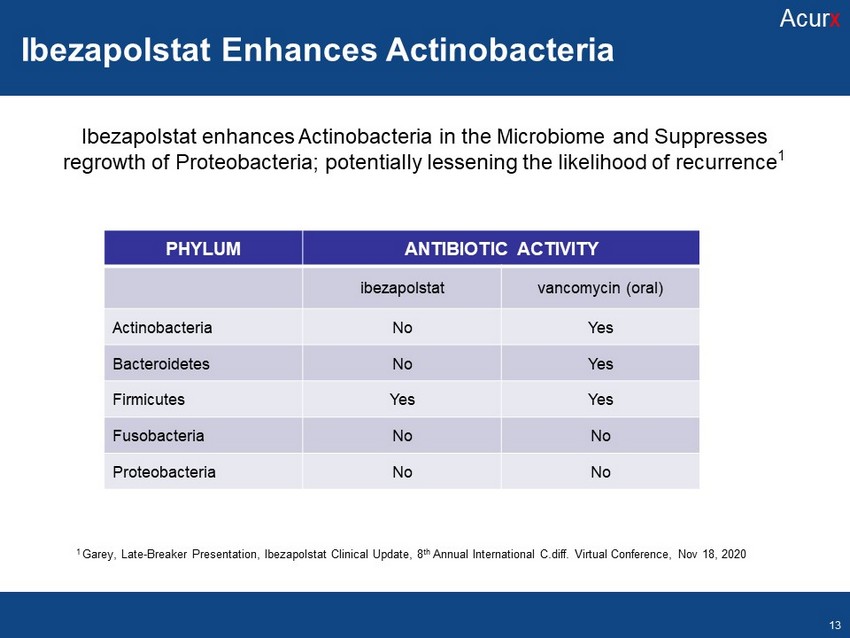
1 Garey, Late - Breaker Presentation, Ibezapolstat Clinical Update, 8 th Annual International C . diff . Virtual Conference, Nov 18 , 2020 PHYLUM ANTIBIOTIC ACTIVITY ibezapolstat vancomycin (oral) Actinobacteria No Yes Bacteroidetes No Yes Firmicutes Yes Yes Fusobacteria No No Proteobacteria No No Ibezapolstat enhances Actinobacteria in the Microbiome and Suppresses regrowth of Proteobacteria ; potentially lessening the likelihood of recurrence 1 Ibezapolstat Enhances Actinobacteria Acur x 13
ACX - 375C Highlights Second DNA Pol IIIC Inhibitor ACX - 375C : Oral and I.V. formulation targets treatment of Staphylococcus, Streptococcus and Enterococcal infections , including vancomycin - resistant enterococcus (VRE), Methicillin - resistant staph (MRSA) and other resistant G+ bacterial infections; WHO/CDC Priority Pathogen List 1 ▪ In hospitalized patients, MRSA accounted for 52% of all infections, almost twice as many as MDR Gram - negative infections 2 Potential Clinical Indications : Urinary tract, hospital acquired catheter/blood stream, intra - abdominal, skin/soft tissue, bone/joint, pneumonia, ear/sinus infections Unmet Medical Need : Antibiotic resistance to currently used antibiotics; including daptomycin and linezolid - resistant bacteria 3 IP and Regulatory : 2 patents covering composition - of - matter, formulation and method - of - use expire December 2039. QIDP, Fast Track and NCE eligible (potential regulatory exclusivity 10 years from FDA approval). Disclosures Disclosures 1 . CDC Antibiotic Resistance Threats in the U . S . , 2019 , Atlanta, U . S . Department of Health and Human Services, CDC, Nov . 2019 2 . Jernigan, et al . , Multidrug - Resistant Bacterial Infections in U . S . Hospitalized Patients, 2012 – 2017 , N Engl J Med 382 : 1309 - 19 ; ( 2020 ) 3 . Wei - Chu Xu, et al . , Bioorganic & Medicinal Chemistry https : //doi . org/ 10 . 1016 /j . bmc . 2019 . 06 . 017 ACX - 375C Highlights Acur x 14
Microbiome comparison to vancomycin Vancomycin ▪ 2 to 3 log reduction in healthy bacteria Ibezapolstat ▪ No appreciable reduction ▪ Poorly soluble in the gut ▪ Free concentrations of ibezapolstat high enough, we believe, to kill C. difficile but too low, we believe, to kill healthy bacteria like Bacteroides & Firmicutes which constitute ~ 90% of the healthy microbiome Disclosures Disclosures Novel ACX - 375 Analogs: Antibacterial Agents Targeting DNA Pol III 1 . Press Release . Acurx’s Novel Lead Antibiotic Candidate Presented at Two Prominent International Scientific Conferences . Oct 2019 . Acur x Microbiome: Ibezapolstat vs. Vancomycin Acur x 15 Head - to - Head Comparison In Phase 1 clinical trial 1 :
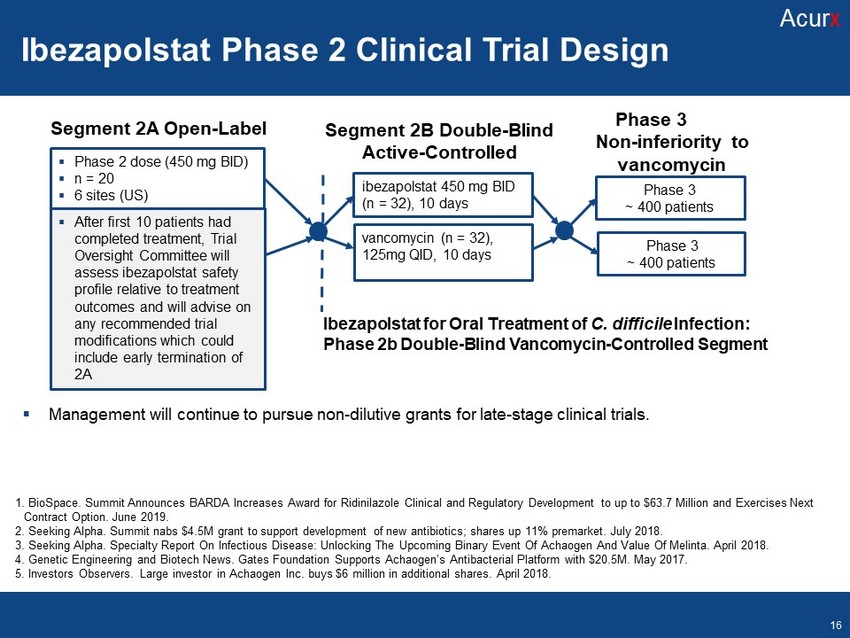
Ibezapolstat Ph 2, Ph 3 Clinical Trial Design Segment 2A Open - Label Segment 2B Double - Blind Active - Controlled ▪ Phase 2 dose (450 mg BID) ▪ n = 20 ▪ 6 sites (US) ▪ After first 10 patients had completed treatment, Trial Oversight Committee will assess ibezapolstat safety profile relative to treatment outcomes and will advise on any recommended trial modifications which could include early termination of 2A ibezapolstat 450 mg BID (n = 32), 10 days vancomycin (n = 32), 125mg QID, 10 days Phase 3 ~ 400 patients Ibezapolstat for Oral Treatment of C. difficile Infection: Phase 2b Double - Blind Vancomycin - Controlled Segment Phase 3 Non - inferiority to vancomycin Phase 3 ~ 400 patients ▪ Management will continue to pursue non - dilutive grants for late - stage clinical trials. Disclosures Disclosures Novel ACX - 375 Analogs: Antibacterial Agents Targeting DNA Pol IIIC ACX - 362E Highlights Disclosures Disclosures Microbiome comparison to vancomycin Disclosures 1. BioSpace . Summit Announces BARDA Increases Award for Ridinilazole Clinical and Regulatory Development to up to $63.7 Million and Exercises Next Contract Option. June 2019. 2. Seeking Alpha. Summit nabs $4.5M grant to support development of new antibiotics; shares up 11% premarket. July 2018. 3. Seeking Alpha. Specialty Report On Infectious Disease: Unlocking The Upcoming Binary Event Of Achaogen And Value Of Melinta . April 2018. 4. Genetic Engineering and Biotech News. Gates Foundation Supports Achaogen’s Antibacterial Platform with $20.5M. May 2017. 5. Investors Observers. Large investor in Achaogen Inc. buys $6 million in additional shares. April 2018. Ibezapolstat Phase 2 Clinical Trial Design Acur x 16
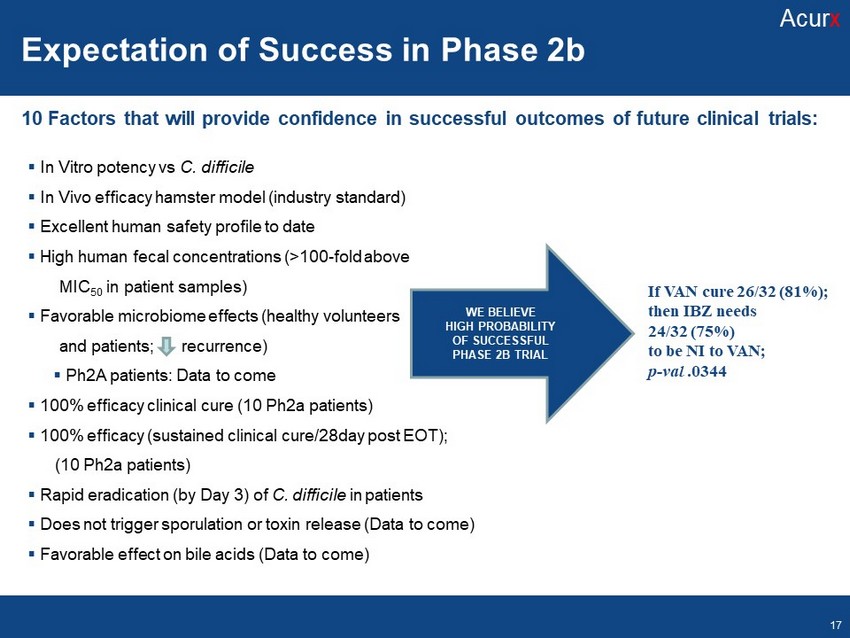
▪ In Vitro potency vs C. difficile ▪ In Vivo efficacy hamster model (industry standard) ▪ Excellent human safety profile to date ▪ High human fecal concentrations (>100 - fold above MIC 50 in patient samples) ▪ Favorable microbiome effects (healthy volunteers and patients; recurrence) ▪ Ph2A patients: Data to come ▪ 100% efficacy clinical cure (10 Ph2a patients) ▪ 100% efficacy (sustained clinical cure/28day post EOT); (10 Ph2a patients) ▪ Rapid eradication (by Day 3) of C. difficile in patients ▪ Does not trigger sporulation or toxin release (Data to come) ▪ Favorable effect on bile acids (Data to come) WE BELIEVE HIGH PROBABILITY OF SUCCESSFUL PHASE 2B TRIAL If VAN cure 26/32 (81%); then IBZ needs 24/32 (75%) to be NI to VAN; p - val .0344 10 Factors that will provide confidence in successful outcomes of future clinical trials: Expectation of Success in Phase 2b Acur x 17

Disclosures There is no guarantee that any specific objective will be achieved. Acurx Pharmaceuticals, LLC products are in development, a nd there is no guarantee that this development will have a successful outcome. Clinical trials are in the early stages. Pre - Clinical trials are tested on animals. Investments may be illiquid, highly speculative and there is risk of the total loss of your investment. See disclosures at the beginning. 18 External positive drivers in 2020 encouraging for sector PASTEUR Act ▪ P rovide critical ‘pull’ incentive in US ▪ HHS to p ay subscription payment for eligible products ▪ $ 750m - $ 3bn subscription payments over 10 years ▪ Transitional support available for Ph3 trials and manufacturing AMR Action Fund ▪ Cross - industry collaborative investment initiative ▪ P rovide $1b n support for clinical activities ▪ Currently in formation and ready for first investments in 2021 ▪ Acurx eligible for support and is engaged with the fund DISARM ACT EU Pull Incentives ▪ R emove s financial disincentive to prescribe novel agents ▪ Provides clinicians greater opportunity to treat infectious diseases with most effective agents ▪ Allows novel agents to be reimbursed in the hospital setting outside constraint of the DRG ▪ New Pharmaceutical Strategy for Europe specifically discusses antibiotics ▪ New European pull - incentives under discussion ▪ New European " BARDA - like " body (Health Emergency Response Authority) to be created External Positive Drivers in 2020 Acur x 18

Experienced Senior Executive Management ▪ David P. Luci, CPA, Esq., Former CEO Dipexium Pharmaceuticals ( Nasdaq:DPRX ); Abeona Therapeutics, MacroChem , Bioenvision . Raised capital in several public offerings and private placements; sold 3 public companies from “C” suite ▪ Robert J. DeLuccia, Former Chairman Dipexium Pharmaceuticals (Nasdaq: DPRX); Former President Sanofi U.S.and Pfizer, Sr. Executive; Former CEO Immunomedics (Nasdaq: IMMU) and MacroChem Corporation (OTC BB: MACM); Lead Director BOD, IBEX Pharmaceuticals (IBT - TSX) Disclosures Disclosures Novel ACX - 375 Analogs: Antibacterial Agents Targeting DNA Pol IIIC ACX - 362E Highlights Disclosures Disclosures Experienced Management Acur x 19

Experienced Senior Management Team ▪ Michael Silverman , MD, FACP, Medical Director KPMG Health Care Consulting, Biopure Corp, Sandoz, Sterling - Winthrop (Kodak - Sanofi) and clinical practice of medicine ▪ Les Johnson , Manufacturing Director Clear Path Development, Salamandra , Celsis , Cambrex , Biosynexus , Baxter Bioscience, Protein Polymer Technologies, Bayer Biologics, Cetus/Codon/ Berlex ▪ Xiang Yu , Ph.D., Director of Pre - Clinical Development Cubist Pharmaceuticals, Accellient Partners, Ironwood Pharmaceuticals, Epix Pharmaceuticals ▪ Larry Mortin , Ph.D., Director of Pharmacology Cubist Pharmaceuticals Disclosures Disclosures ACX - 362E Highlights Disclosures Disclosures Management Team Acur x 1 20
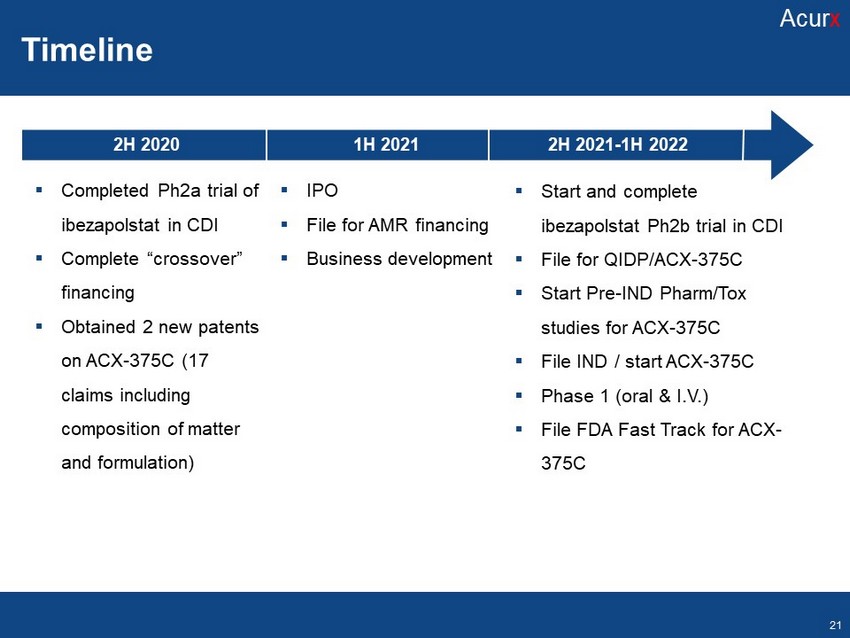
Timeline Disclosures Disclosures Novel ACX - 375 Analogs: Antibacterial Agents Targeting DNA Pol IIIC ACX - 362E Highlights Disclosures Disclosures Microbiome comparison to vancomycin Disclosures 1H 2021 2H 2020 2H 2021 - 1H 2022 ▪ Completed Ph2a trial of ibezapolstat in CDI ▪ Complete “crossover” financing ▪ Obtained 2 new patents on ACX - 375C (17 claims including composition of matter and formulation) ▪ Start and complete ibezapolstat Ph2b trial in CDI ▪ File for QIDP/ACX - 375C ▪ Start Pre - IND Pharm/Tox studies for ACX - 375C ▪ File IND / start ACX - 375C ▪ Phase 1 (oral & I.V.) ▪ File FDA Fast Track for ACX - 375C ▪ IPO ▪ File for AMR financing ▪ Business development Timeline Acur x 21

Disclosures Disclosures ACX - 362E Highlights Disclosures Disclosures Microbiome comparison to vancomycin Disclosures Christopher Carlin Alexander Capital, LP ccarlin@alexandercapitallp.com (646) 787 - 8890 June 2021