

Advancing a New Class of Antibiotics to Phase 3 Trials Targeting “Priority Pathogens” David P. Luci, President & CEO April 2024 – WHO and CDC Issuer Free Writing Prospectus Filed Pursuant to Rule 433 Registration Statement No. 333 - 278028 April 2, 2024

Disclosure 1 FREE WRITING PROSPECTUS This presentation highlights basic information about Acurx Pharmaceuticals, Inc. (the ”Company”) and the offering. Because it is a summary that has been prepared solely for informational purposes, it does not contain all of the information that you should consider before investing in our Company. Exc ept as otherwise indicated, this presentation speaks only as of the date hereof. This presentation does not constitute an offer to sell, nor a solicitation of an offer to buy, these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. by any person in an y jurisdiction in which it is unlawful for such person to make such an offering or solicitation. Neither the Securities and Exchange Commission (“SEC”) nor any other regulatory body has ap pro ved or disapproved of the securities or passed upon the accuracy or adequacy of this presentation. Any representation to the contrary is a criminal offense. This presentation includes industry and market data that we obtained from industry publications and journals, third - party studie s and surveys, internal company studies and surveys, and other publicly available information. Industry publications and surveys generally state that the information contained t her ein has been obtained from sources believed to be reliable. Although we believe the industry and market data to be reliable as of the date of this presentation, this informat ion could prove to be inaccurate. Industry and market data could be wrong because of the method by which sources obtained their data and because information cannot always be verified w ith complete certainty due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncert ain ties. In addition, we do not know all of the assumptions that were used in preparing the forecasts from the sources relied upon or cited herein. A registration statement on Form S - 1 (File No. 333 - 278028), as amended, including a preliminary prospectus, relating to the off ering of securities has been filed by the Company with the SEC. The registration statement has not yet become effective. Before you invest, you should read the preliminary prospec tus in the registration statement and, when available, the final prospectus relating to the offering. An electronic copy of the preliminary prospectus relating to the offering is avail abl e, and a copy of the final prospectus relating to the offering will be available, on the website of the SEC at www.sec.gov. Copies of the preliminary prospectus and final prospectus relat ing to the offering, when available, may be obtained by contacting Titan Partners Group, LLC, a division of American Capital Partners, LLC, 4 World Trade Center, 29th Floor, New Yo rk, New York 10007, by phone at (929) 833 - 1246 or by email at info@titanpartnersgroup.com .

Disclosure (cont’d.) FORWARD LOOKING STATEMENTS This presentation contains forward - looking statements as defined in the Private Securities Litigation Reform Act of 1995 regardi ng, among other things, development plans, regulatory activities, anticipated milestones, product candidate benefits, competitive position, business strategies, objectives of mana gem ent, potential growth opportunities, potential market size, possible or assumed future results of operations, projected costs and use of proceeds. In some cases, forward - looking statements can be identified by terms such as “may,” “will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “could,” “intent,” “target,” “project,” “contemplate,” “believe,” “estimate, ” “ predict,” “potential” or “continue” or the negative of these terms or other similar expressions. All statements other than statements of historical facts contained in this presentation are forward - looking statements. The Company may not actually achieve the plans, intentions or expectations disclosed in these forward - looking statements. Actual results or events could dif fer materially from the plans, intentions and expectations disclosed in these forward - looking statements as a result of various factors, including: uncertainties inherent in the initiati on and completion of preclinical studies and clinical trials and clinical development of the Company’s product candidates, including adverse results in our clinical development processes; wh eth er results from one clinical trial will be predictive of the results of future trials and whether preliminary data from our clinical trials will be predictive of final results from such tri als; decisions made by the U.S. Food and Drug Administration and other regulatory authorities with respect to the development and commercialization of our products; availability of funding s uff icient for the Company’s foreseeable and unforeseeable operating expenses and capital expenditure requirements; our ability to obtain, maintain and enforce intellectual property an d o ther proprietary rights for our product candidates; our ability to implement our strategic plans; and other factors discussed in the “Risk Factors” section of the Company’s filings wit h the SEC, including the Company’s Annual Report on Form 10 - K for the fiscal year ended December 31, 2023, filed with the SEC on March 15, 2024. The forward - looking statements included in this presentation represent the Company’s views as of the date of this presentation. The Company anticipates that subsequent events and developments will cause its views to cha nge . However, while the Company may elect to update these forward - looking statements at some point in the future, it specifically disclaims any obligation to do so unless required by applicable securities laws. These forward - looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this presen tat ion. 2
3 Offering Summary Issuer Ticker Exchange Proceeds Overallotment Option Sole Bookrunner Acurx Pharmaceuticals ACXP Nasdaq $25m 15% Titan Partners Group LLC, a division of American Capital Partners, LLC Use of Proceeds: Working capital and other general purposes, including, but not limited to, clinical trials, research and development activities, acquisitions and collaborations. Securities Offered Common Stock
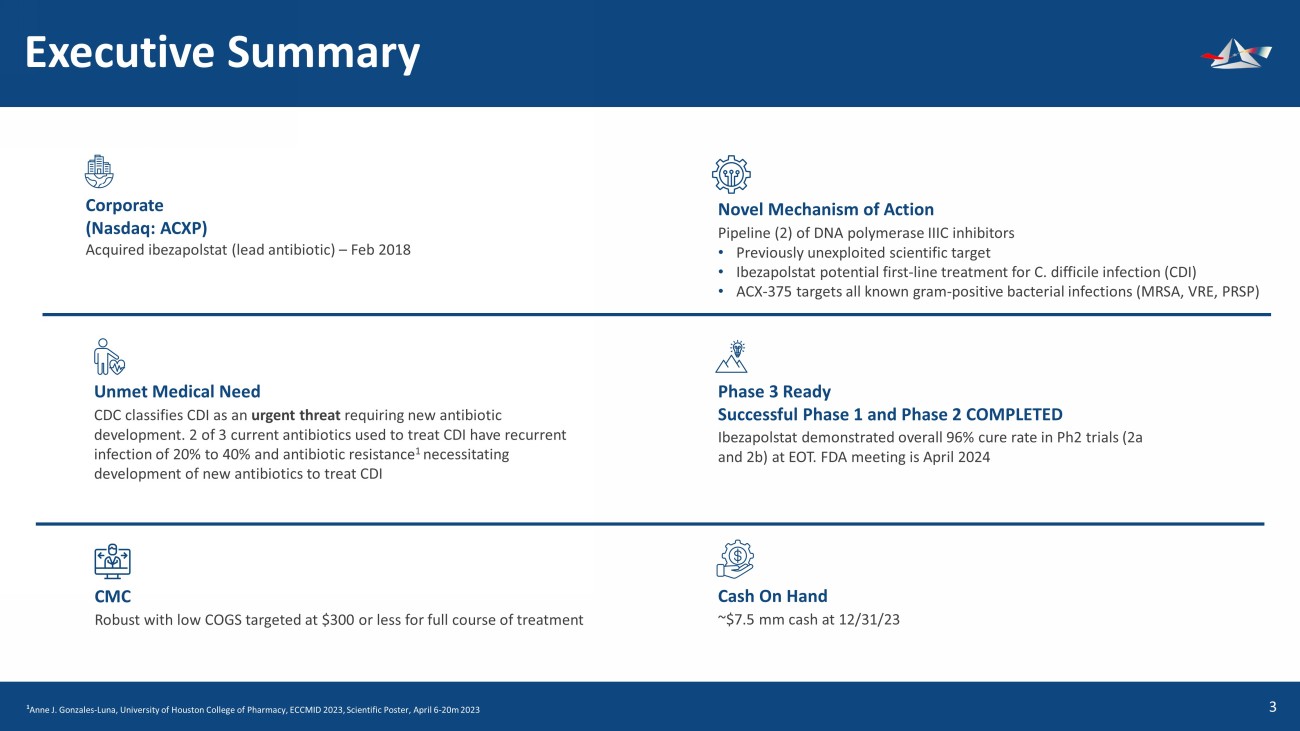
&UHDWHGE\%(-281 IURPWKH1RXQ3URMHFW &UHDWHGE\(NR3XUQRPR IURPWKH1RXQ3URMHFW &UHDWHGE\177 IURPWKH1RXQ3URMHFW &UHDWHGE\.LUDQ6KDVWU\ IURPWKH1RXQ3URMHFW Executive Summary 4 Corporate (Nasdaq: ACXP) Acquired ibezapolstat (lead antibiotic) – Feb 2018 &UHDWHGE\NDNKLP IURP1RXQ3URMHFW Unmet Medical Need CDC classifies CDI as an urgent threat requiring new antibiotic development. 2 of 3 current antibiotics used to treat CDI have recurrent infection of 20% to 40% and antibiotic resistance 1 necessitating development of new antibiotics to treat CDI CMC Robust with low COGS targeted at $300 or less for full course of treatment Novel Mechanism of Action Pipeline (2) of DNA polymerase IIIC inhibitors • Previously unexploited scientific target • Ibezapolstat potential first - line treatment for C. difficile infection (CDI) • ACX - 375 targets all known gram - positive bacterial infections (MRSA, VRE, PRSP) Phase 3 Ready Successful Phase 1 and Phase 2 COMPLETED Ibezapolstat demonstrated overall 96% cure rate in Ph2 trials (2a and 2b) at EOT. FDA meeting is April 2024 Cash On Hand ~$7.5 mm cash at 12/31/23 1 Anne J. Gonzales - Luna, University of Houston College of Pharmacy, ECCMID 2023, Scientific Poster, April 6 - 20m 2023 &UHDWHGE\.DQ.LQJSHWFKDUDW IURPWKH1RXQ3URMHFW
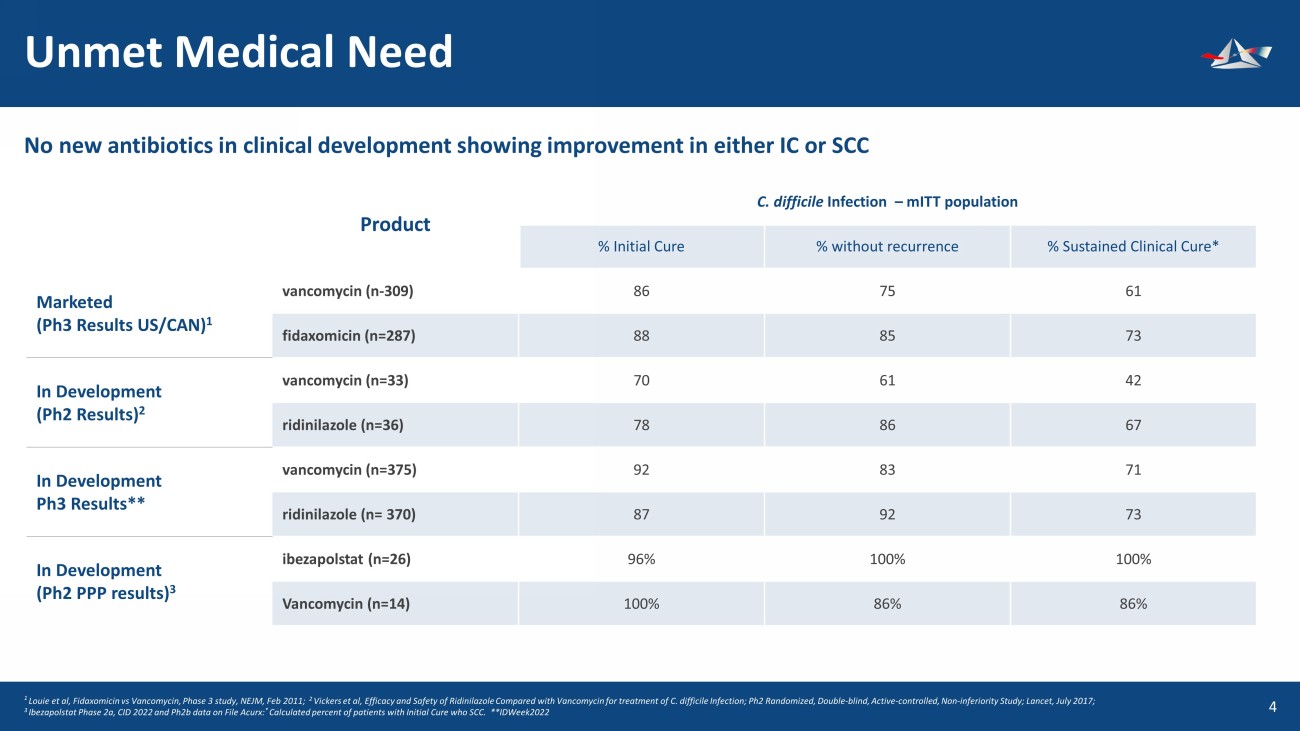
Unmet Medical Need 5 1 Louie et al, Fidaxomicin vs Vancomycin, Phase 3 study, NEJM, Feb 2011; 2 Vickers et al, Efficacy and Safety of Ridinilazole Compared with Vancomycin for treatment of C. difficile Infection; Ph2 Rand omi zed, Double - blind, Active - controlled, Non - inferiority Study; Lancet, July 2017; 3 Ibezapolstat Phase 2a, CID 2022 and Ph2b data on File Acurx: * Calculated percent of patients with Initial Cure who SCC. **IDWeek2022 No new antibiotics in clinical development showing improvement in either IC or SCC Product C. difficile Infection – mITT population % Initial Cure % without recurrence % Sustained Clinical Cure* Marketed (Ph3 Results US/CAN) 1 vancomycin (n - 309) 86 75 61 fidaxomicin (n=287) 88 85 73 In Development (Ph2 Results) 2 vancomycin (n=33) 70 61 42 ridinilazole (n=36) 78 86 67 In Development Ph3 Results** vancomycin (n=375) 92 83 71 ridinilazole (n= 370) 87 92 73 In Development (Ph2 PPP results) 3 ibezapolstat (n=26) 96% 100% 100% Vancomycin (n=14) 100% 86% 86%
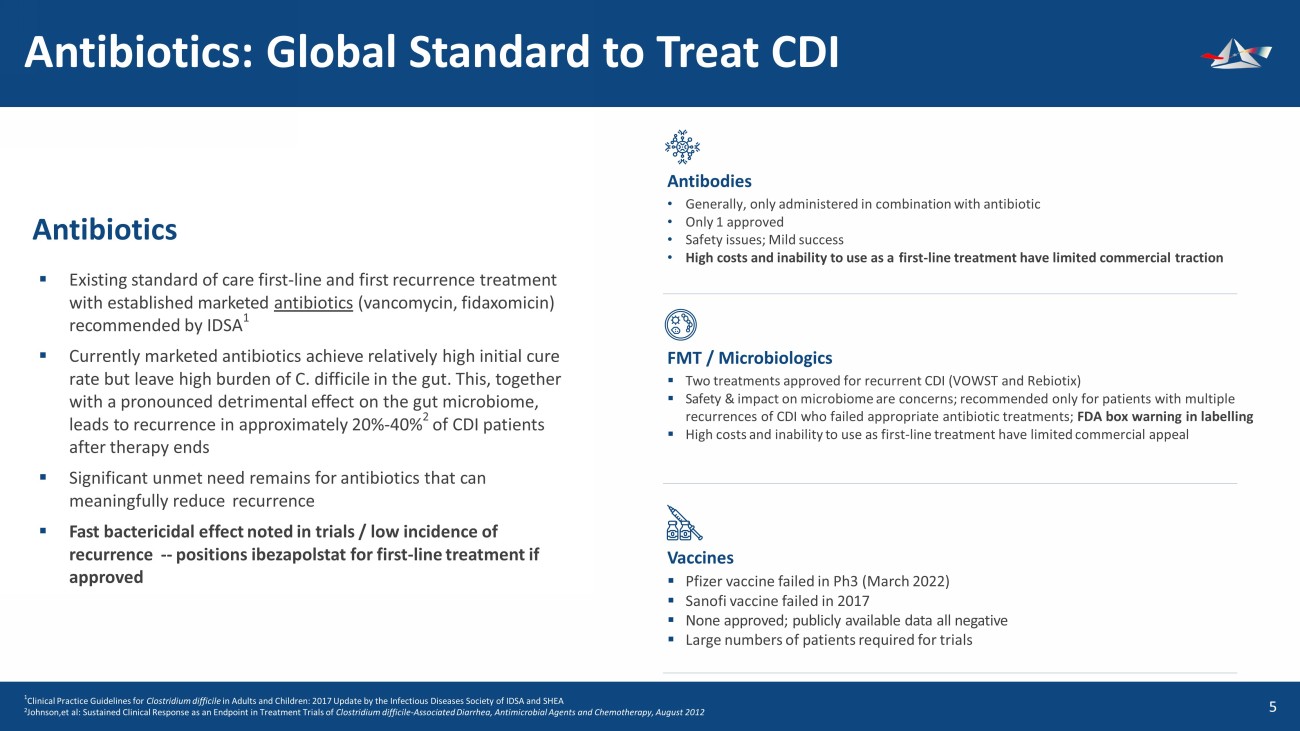
&UHDWHGE\QDUHHUDWMDLNDHZ IURPWKH1RXQ3URMHFW &UHDWHGE\7HPSODWH IURPWKH1RXQ3URMHFW Antibiotics: Global Standard to Treat CDI 6 Antibiotics ▪ Existing standard of care first - line and first recurrence treatment with established marketed antibiotics (vancomycin, fidaxomicin) recommended by IDSA 1 ▪ Currently marketed antibiotics achieve relatively high initial cure rate but leave high burden of C. difficile in the gut. This, together with a pronounced detrimental effect on the gut microbiome, leads to recurrence in approximately 20% - 40% 2 of CDI patients after therapy ends ▪ Significant unmet need remains for antibiotics that can meaningfully reduce recurrence ▪ Fast bactericidal effect noted in trials / low incidence of recurrence -- positions ibezapolstat for first - line treatment if approved Antibodies • Generally, only administered in combination with antibiotic • Only 1 approved • Safety issues; Mild success • High costs and inability to use as a first - line treatment have limited commercial traction FMT / Microbiologics ▪ Two treatments approved for recurrent CDI (VOWST and Rebiotix) ▪ Safety & impact on microbiome are concerns; recommended only for patients with multiple recurrences of CDI who failed appropriate antibiotic treatments; FDA box warning in labelling ▪ High costs and inability to use as first - line treatment have limited commercial appeal Vaccines ▪ Pfizer vaccine failed in Ph3 (March 2022) ▪ Sanofi vaccine failed in 2017 ▪ None approved; publicly available data all negative ▪ Large numbers of patients required for trials 1 Clinical Practice Guidelines for Clostridium difficile in Adults and Children: 2017 Update by the Infectious Diseases Society of IDSA and SHEA 2 Johnson,et al: Sustained Clinical Response as an Endpoint in Treatment Trials of Clostridium difficile - Associated Diarrhea, Antimicrobial Agents and Chemotherapy, August 2012 &UHDWHGE\(XFDO\S IURPWKH1RXQ3URMHFW
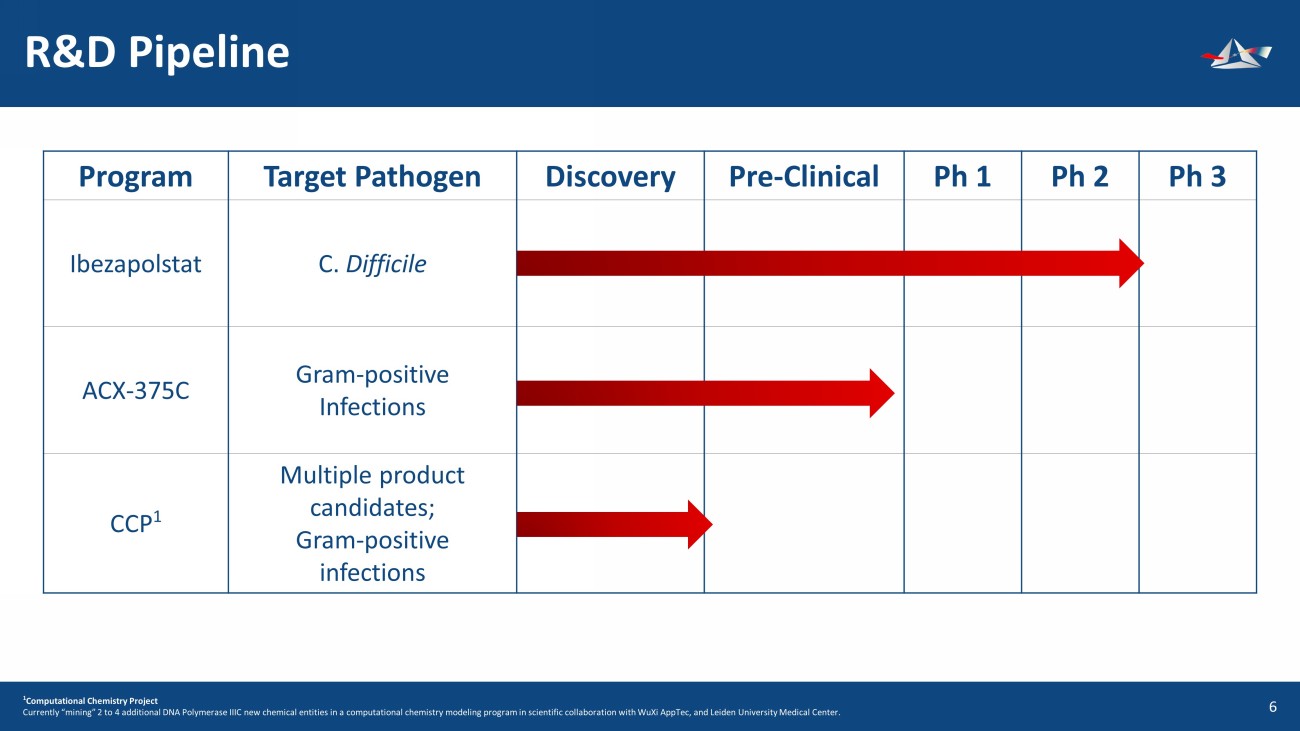
Program Target Pathogen Discovery Pre - Clinical Ph 1 Ph 2 Ph 3 Ibezapolstat C. Difficile ACX - 375C Gram - positive Infections CCP 1 Multiple product candidates; Gram - positive infections R&D Pipeline 7 1 Computational Chemistry Project Currently “mining” 2 to 4 additional DNA Polymerase IIIC new chemical entities in a computational chemistry modeling program in scientific collaboration with WuXi AppTec, and Leiden University Medical Center.
Recent & Upcoming Milestones 8 Clinical Lead AB treating CDI - recently completed Ph2b trial ▪ Dec ‘23 : Sustained Clinical Cure Data (30 days after EOT) ▪ Jan ’24: Extended Clinical Cure Data (94 days out) ▪ Jan ‘24 : Microbiome head - to - head comparison to standard of vancomycin Clinical, Regulatory WW Strategy ▪ Q2 ’24: FDA Meeting – finalize Ph3 mandate ▪ 2H ‘24: Launch international strategy for commercialization in EU, UK, Japan & CDN ▪ File for FDA QIDP Designation for ACX - 375 ▪ Enroll first patient in Ph3 trial ▪ Commence IND - enabling preclinical tox studies for ACX - 375 Manufacturing ▪ Scaled up to 10 kg batches of API to be finalized ▪ “Made in the USA” policy launch for fill/finish &UHDWHGE\DILDQURF IURPWKH1RXQ3URMHFW x &UHDWHGE\JO\SK IDLVDORYHUV IURPWKH1RXQ3URMHFW
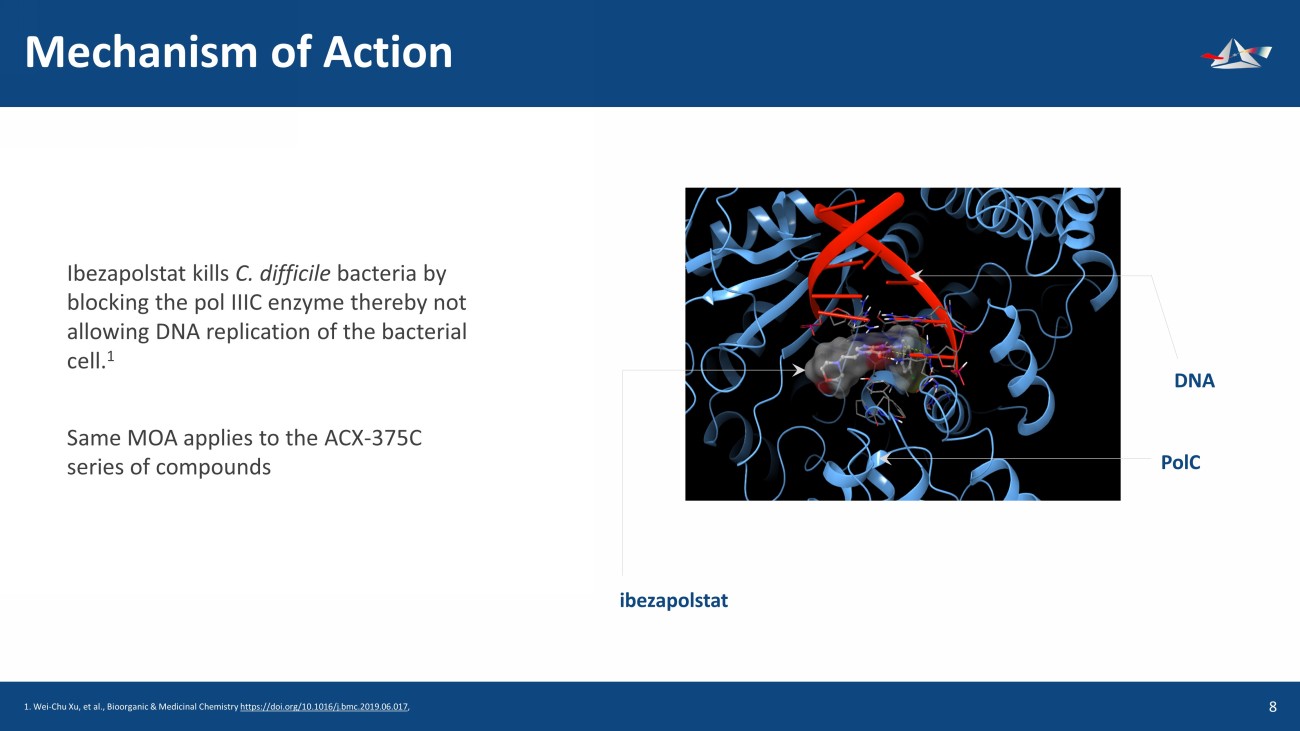
Mechanism of Action 9 Ibezapolstat kills C. difficile bacteria by blocking the pol IIIC enzyme thereby not allowing DNA replication of the bacterial cell. 1 Same MOA applies to the ACX - 375C series of compounds ibezapolstat DNA PolC 1. Wei - Chu Xu, et al., Bioorganic & Medicinal Chemistry https://doi.org/10.1016/j.bmc.2019.06.017 ,
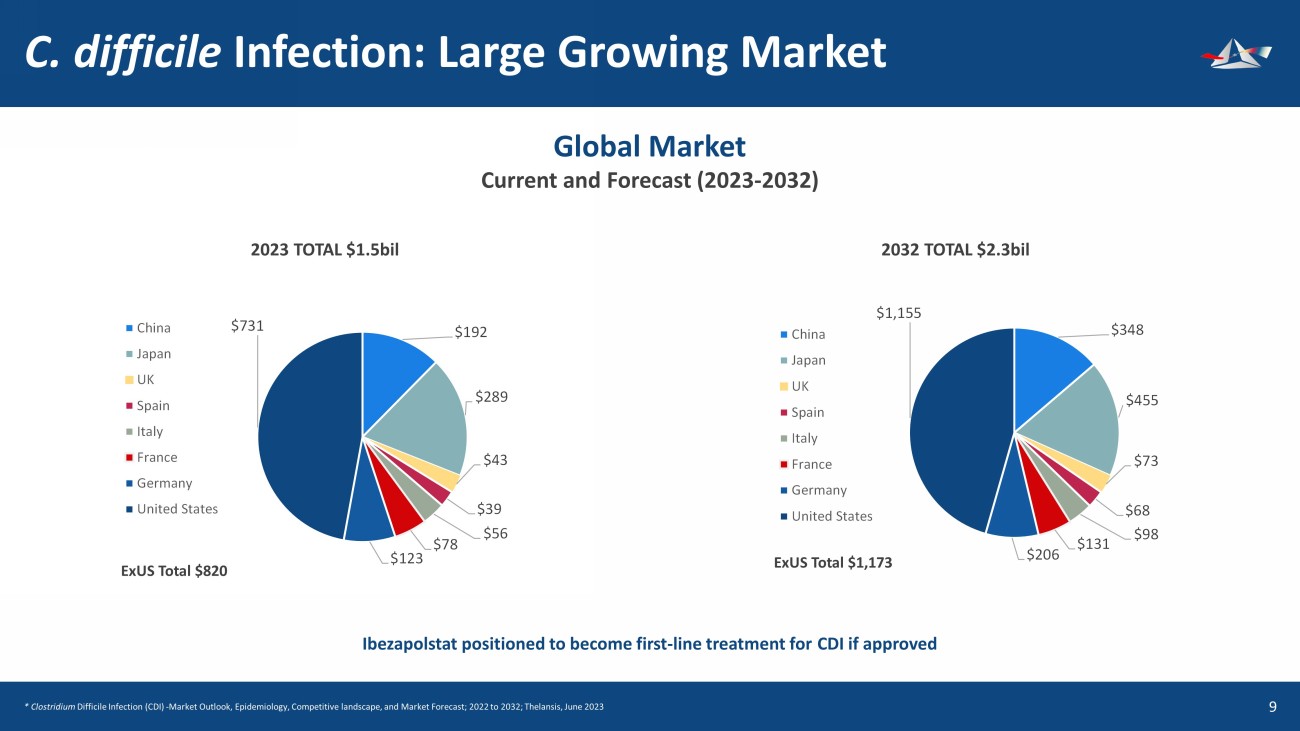
C. difficile Infection: Large Growing Market 10 $192 $289 $43 $39 $56 $78 $123 $731 China Japan UK Spain Italy France Germany United States $348 $455 $73 $68 $98 $131 $206 $1,155 China Japan UK Spain Italy France Germany United States Global Market Current and Forecast (2023 - 2032) 2023 TOTAL $1.5bil 2032 TOTAL $2.3bil ExUS Total $820 ExUS Total $1,173 Ibezapolstat positioned to become first - line treatment for CDI if approved * Clostridium Difficile Infection (CDI) - Market Outlook, Epidemiology, Competitive landscape, and Market Forecast; 2022 to 2032; Thelansis, J une 2023
Ibezapolstat: Phase 2 Success 11 EOT (End of Treatment) 25/26 (96%) of evaluable patients were cured at EOT (10 of 10 in Ph2a and 15 of 16 in Ph2b). No drug related SAE’s SCC (Sustained Clinical Cures) All 15 ibezapolstat - treated patients in Phase 2b who achieved Clinical Cure (CC) at end of treatment (EOT) remained free of CDI recurrence 30 days after EOT, for a Sustained Clinical Cure (SCC) rate of 100% ECC (Extended Clinical Cures) 100% (5 of 5) of ibezapolstat - treated patients experienced no recurrence of infection Regulatory/Patent Exclusivity Rolling 10 years regulatory exclusivity from FDA approval (QIDP and NCE); similar regulatory exclusivity in EU and internationally; Patents expire September 2030 Clinical Comparability Ph 2 clinical results* shows early - stage clinical comparability of a new class of antibiotics to treat CDI compared to oral vancomycin Ibezapolstat outperformed vancomycin showing eradication of fecal C. difficile at Day 3 of treatment in 15 of 16 treated patients (94%), versus vancomycin which had eradication of C. difficile in 10 of 14 treated patients (71%) Ibezapolstat microbiome head - to - head showed IBZ beat vancomycin at preservation and regrowth of key gut microbiota essential to avoid recurrent CDI CID, 2022 &UHDWHGE\DILDQURF IURPWKH1RXQ3URMHFW &UHDWHGE\.DPLQ*LQNDHZ IURPWKH1RXQ3URMHFW &UHDWHGE\DILDQURF IURPWKH1RXQ3URMHFW
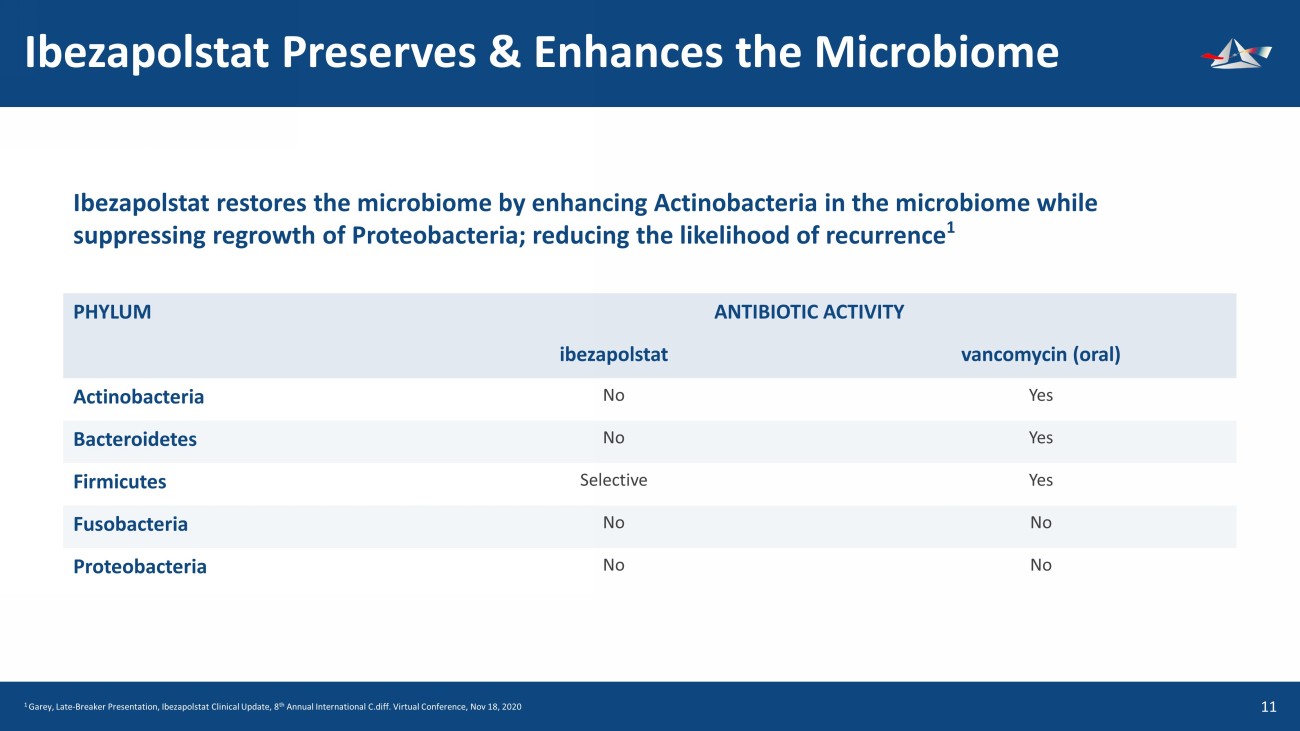
Ibezapolstat Preserves & Enhances the Microbiome 12 Ibezapolstat restores the microbiome by enhancing Actinobacteria in the microbiome while suppressing regrowth of Proteobacteria ; reducing the likelihood of recurrence 1 PHYLUM ANTIBIOTIC ACTIVITY ibezapolstat vancomycin (oral) Actinobacteria No Yes Bacteroidetes No Yes Firmicutes Selective Yes Fusobacteria No No Proteobacteria No No 1 Garey, Late - Breaker Presentation, Ibezapolstat Clinical Update, 8 th Annual International C.diff. Virtual Conference, Nov 18, 2020
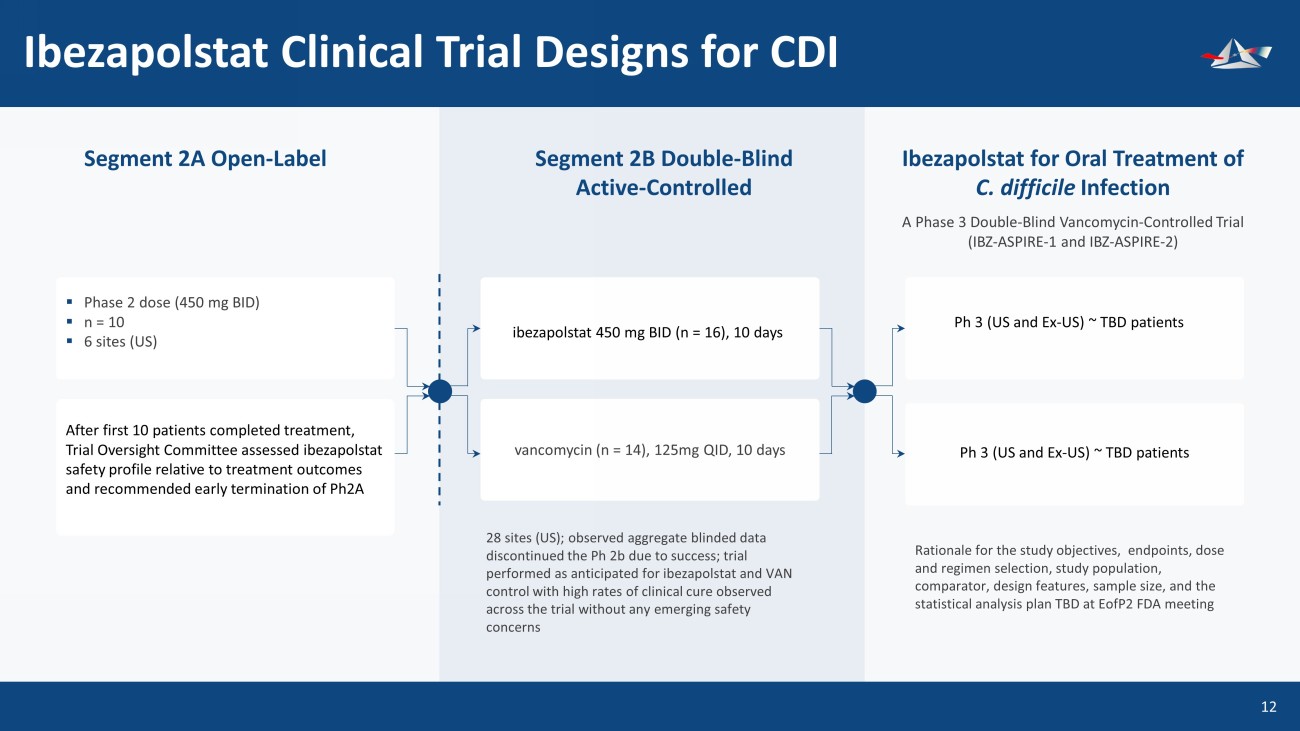
Ibezapolstat Clinical Trial Designs for CDI 13 Segment 2A Open - Label Segment 2B Double - Blind Active - Controlled A Phase 3 Double - Blind Vancomycin - Controlled Trial (IBZ - ASPIRE - 1 and IBZ - ASPIRE - 2) Ibezapolstat for Oral Treatment of C. difficile Infection ▪ Phase 2 dose (450 mg BID) ▪ n = 10 ▪ 6 sites (US) After first 10 patients completed treatment, Trial Oversight Committee assessed ibezapolstat safety profile relative to treatment outcomes and recommended early termination of Ph2A ibezapolstat 450 mg BID (n = 16), 10 days Ph 3 (US and Ex - US) ~ TBD patients 28 sites (US); observed aggregate blinded data discontinued the Ph 2b due to success; trial performed as anticipated for ibezapolstat and VAN control with high rates of clinical cure observed across the trial without any emerging safety concerns Rationale for the study objectives, endpoints, dose and regimen selection, study population, comparator, design features, sample size, and the statistical analysis plan TBD at EofP2 FDA meeting vancomycin (n = 14), 125mg QID, 10 days Ph 3 (US and Ex - US) ~ TBD patients
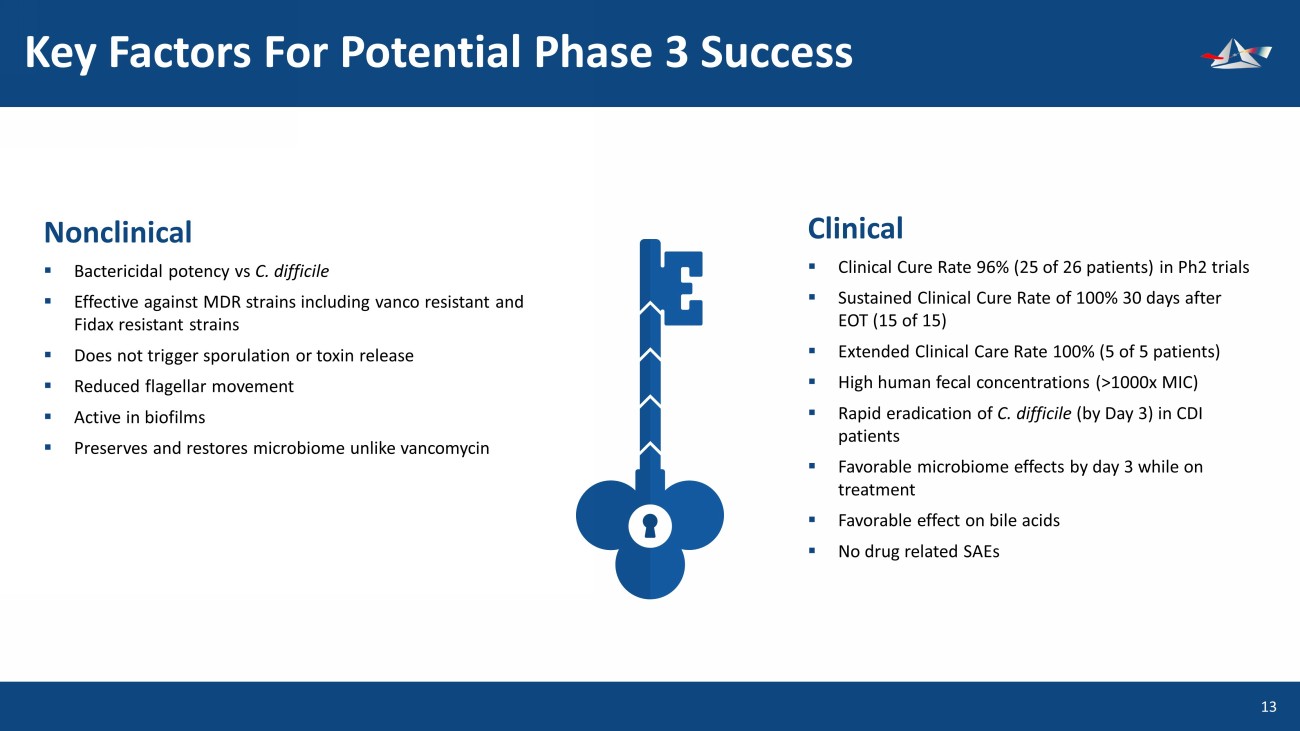
Key Factors For Potential Phase 3 Success 14 Nonclinical ▪ Bactericidal potency vs C. difficile ▪ Effective against MDR strains including vanco resistant and Fidax resistant strains ▪ Does not trigger sporulation or toxin release ▪ Reduced flagellar movement ▪ Active in biofilms ▪ Preserves and restores microbiome unlike vancomycin Clinical ▪ Clinical Cure Rate 96% (25 of 26 patients) in Ph2 trials ▪ Sustained Clinical Cure Rate of 100% 30 days after EOT (15 of 15) ▪ Extended Clinical Care Rate 100% (5 of 5 patients) ▪ High human fecal concentrations (>1000x MIC) ▪ Rapid eradication of C. difficile (by Day 3) in CDI patients ▪ Favorable microbiome effects by day 3 while on treatment ▪ Favorable effect on bile acids ▪ No drug related SAEs
ACX - 375C Highlights 15 VRE hospital infections exceeded carbapenem - resistant (CR) Acinetobacter, MDR Pseudomonas aeruginosa and CR Enterobacteriaceae infections combined 2 Second DNA Pol IIIC Inhibitor ACX - 375C Oral and I.V. formulation targets treatment of Staphylococcus, Streptococcus and Enterococcal infections , including vancomycin - resistant enterococcus (VRE), Methicillin - resistant staph (MRSA) and other resistant G+ bacterial infections; WHO/CDC Priority Pathogen List 1 In hospitalized patients, MRSA accounted for 52% of all infections, almost twice as many as MDR Gram - negative infections 2 1. CDC Antibiotic Resistance Threats in the U.S., 2019, Atlanta, U.S. Department of Health and Human Services, CDC, Nov. 2019 2. Jernigan, et al., Multidrug - Resistant Bacterial Infections in U.S. Hospitalized Patients, 2012 – 2017, N Engl J Med 382:1309 - 19; (2020) 1 2
ACX - 375C Highlights 16 Potential Clinical Indications: (QIDP/Fast Track eligible); ABSSSI (MRSA + other G+) Follow - on: community - acquired bacterial pneumonia, hospital and/or ventilator - associated bacterial pneumonia; bacteremia with or w/o infectious endocarditis, bone/joint infections and diabetic foot infections Unmet Medical Need Antibiotic resistance to currently used antibiotics; including daptomycin and linezolid - resistant bacteria 1,2 IP and Regulatory 2 patents covering composition - of - matter, formulation and method - of - use expire December 2039. QIDP, Fast Track and NCE eligible (potential 10 years of market exclusivity). &UHDWHGE\1LWKLQDQ7DWDK IURPWKH1RXQ3URMHFW &UHDWHGE\DILDQURF IURPWKH1RXQ3URMHFW 1. Jernigan, et al., Multidrug - Resistant Bacterial Infections in U.S. Hospitalized Patients, 2012 – 2017, N Engl J Med 382:1309 - 19; (2020) 2. Wei - Chu Xu, et al., Bioorganic & Medicinal Chemistry https://doi.org/10.1016/j.bmc.2019.06.017 &UHDWHGE\1LWKLQDQ7DWDK IURPWKH1RXQ3URMHFW

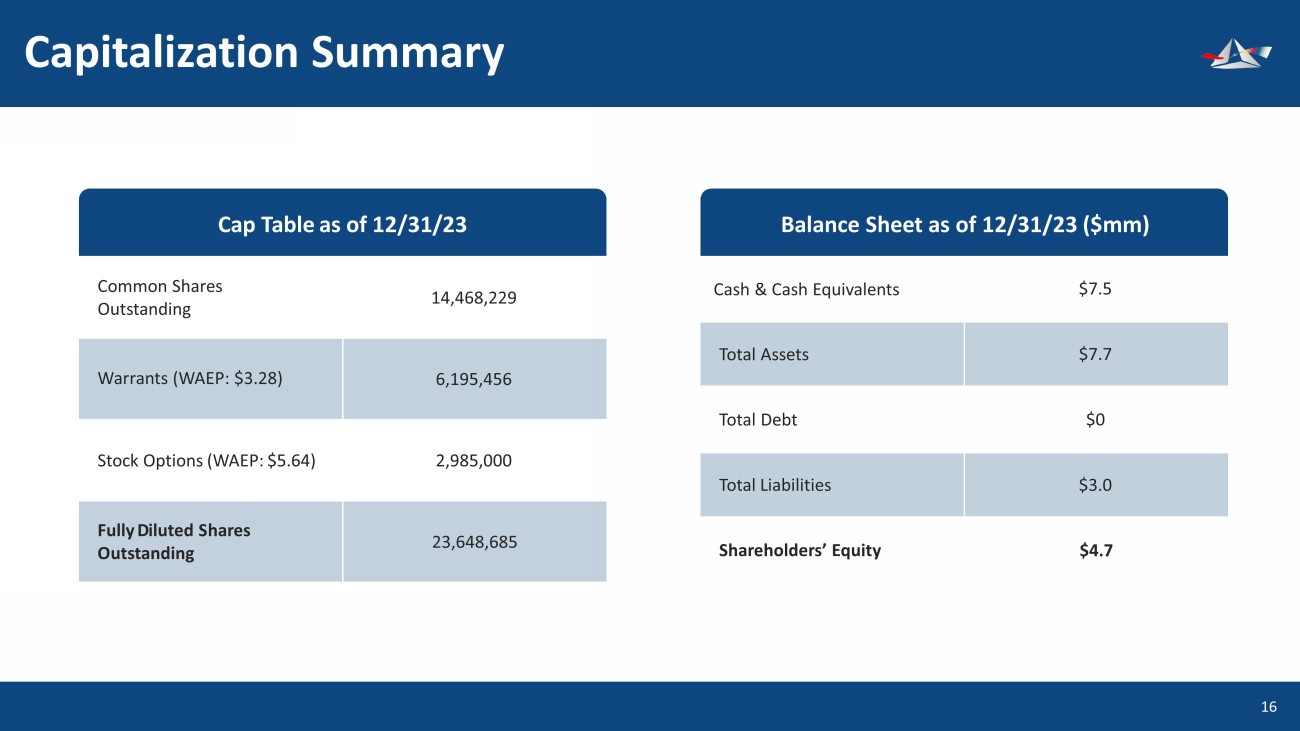
Capitalization Summary 17 Common Shares Outstanding Warrants (WAEP: $3.28) Stock Options (WAEP: $5.64) Fully D iluted S hares O utstanding 14,468,229 6,195,456 2,985,000 23,648,685 Cap Table as of 12/31/23 Cash & Cash Equivalents Total Assets Total Debt Shareholders’ Equity $7.5 $7.7 $0 $4.7 Balance Sheet as of 12/31/23 ($mm) Total Liabilities $3.0
Experienced Senior Executive Management 18 David P. Luci, CPA, Esq Co - Founder & CEO Robert J. DeLuccia Co - Founder & Executive Chairman Robert G. Shawah CPA, Co - Founder & CFO Former CEO of Dipexium Pharmaceuticals (Nasdaq: DPRX), Abeona Therapeutics (Nasdaq: ABEO), MacroChem (OTC BB: MACM), and Bioenvision (Nasdaq: BIVN). Sold all 3 public companies he co - founded or joined in early stage. Orchestrated several in and out - licensing transactions prior to dispositions. M&A and corporate finance attorney (Paul Hastings NY) and CPA with Ernst & Young NY) Former Chairman of Dipexium Pharmaceuticals (Nasdaq: DPRX); Former President Sanofi U.S. and Pfizer, Sr. Executive; Former CEO Immunomedics (Nasdaq: IMMU) and MacroChem Corporation (OTC BB: MACM); Lead Director BOD, IBEX Pharmaceuticals (IBT - TSX) Former Chief Accounting Officer of Dipexium Pharmaceuticals (Nasdaq: DPRX); Former Vice President of Baldwin Pearson & Co., a commercial real estate firm
Strategic Alternatives 19 In parallel with Ph3 trial preparation, explore strategic alternatives OBJECTIVE &UHDWHGE\$OLFH'HVLJQ IURPWKH1RXQ3URMHFW Ph 3 trial preparation PLAN 1A PLAN 1B Partnering / M&A initiative Enroll Ph3 & build out pipeline
Proposed Pathway To Commercialization 20 Current Data Library International Ph3 Trials Regulatory Approvals U.S. Food & Drug Administration Health Canada European Medicines Agency UK Medicines & Healthcare Product Agency Japan: Pharmaceuticals and Medical Devices Agency + =

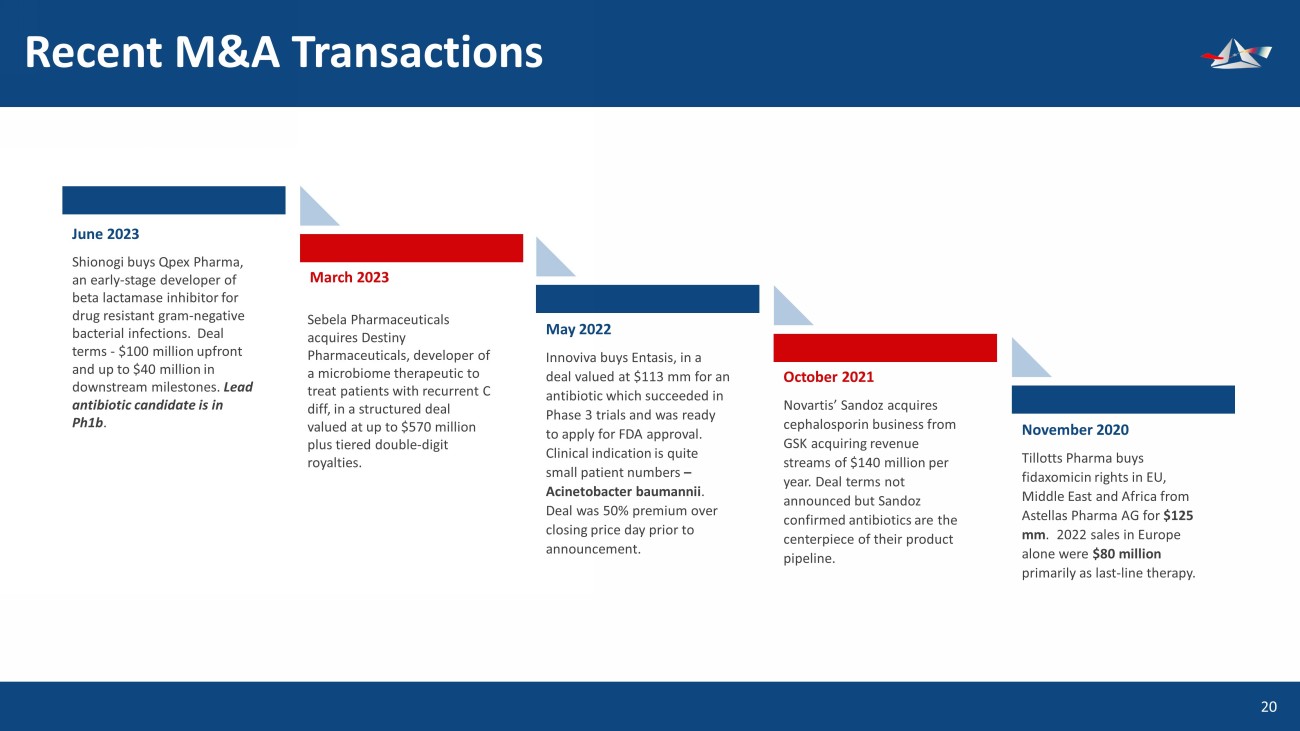
Recent M&A Transactions 21 June 2023 Shionogi buys Qpex Pharma, an early - stage developer of beta lactamase inhibitor for drug resistant gram - negative bacterial infections. Deal terms - $100 million upfront and up to $40 million in downstream milestones. Lead antibiotic candidate is in Ph1b . March 2023 May 202 2 Innoviva buys Entasis, in a deal valued at $113 mm for an antibiotic which succeeded in Phase 3 trials and was ready to apply for FDA approval. Clinical indication is quite small patient numbers – Acinetobacter baumannii . Deal was 50% premium over closing price day prior to announcement. October 2021 Novartis’ Sandoz acquires cephalosporin business from GSK acquiring revenue streams of $140 million per year. Deal terms not announced but Sandoz confirmed antibiotics are the centerpiece of their product pipeline. November 2020 Tillotts Pharma buys fidaxomicin rights in EU, Middle East and Africa from Astellas Pharma AG for $125 mm . 2022 sales in Europe alone were $80 million primarily as last - line therapy. Sebela Pharmaceuticals acquires Destiny Pharmaceuticals, developer of a microbiome therapeutic to treat patients with recurrent C diff, in a structured deal valued at up to $570 million plus tiered double - digit royalties.

Non - Executive Members of the Board of Directors 22 ▪ Jack H. Dean , Ph.D., Former Director, Worldwide Pre - Clinical Research at Sanofi; Research Professor, Univ of Arizona (Pharmacology & Toxicology) ▪ James Donohue , Vice President of Charles River Associates (Nasdaq: CRAI) ▪ Thomas Harrison , Chairman Emeritus of the Diversified Agency Services (“DAS”) division of Omicron Group Inc. (NYSE:OMC). Previous Chairman and Chief Executive Officer of Omicron Group Inc. ▪ Carl Sailer , VP Global Account Lead for Syneos Health (Nasdaq:SYNH). Previous VP of Sales and Marketing for Emisphere Technologies ▪ Joseph C. Scodari , Chairman of the Board of Directors of Optinose (Nasdaq:OPTN). Previous Worldwide Chairman, Pharmaceuticals Group, of Johnson & Johnson, and member of Executive Committee * Co - authors of Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infect ious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). April 2018
Advancing a New Class of Antibiotics to Phase 3 Trials Targeting “Priority Pathogens” David P. Luci, President & CEO April 2024 – WHO and CDC